Editorial Board Comments:
Ian Gilchrist:
I think the important issue is to consider the alternative and potential risks. If I have difficulty reaching the LIMA from the right and the surgeons have already used the left radial, I usually use the left ulnar for the LIMA pictures. While this is a small experience, angiograms of these arteries usually show many collaterals via intraosseous vessels. No ischemic complaints during or after procedure (so far).
Mauricio Cohen:
Please review a publication by Sasko Kedev. He has a series of cases performed via Ulnar approach in patients with occluded radial arteries. Kedev S, Zafirovska B, Dharma S, Petkoska D. Safety and feasibility of transulnar catheterization when ipsilateral radial access is not available. Catheter Cardiovasc Interv. 2014;83:E51-60. I have switched to ipsilateral ulnar after finding a non-negotiable radial loop. The patient had a sheath in the radial and ulnar arteries simultaneously and there was no ischemia. Before getting access I the ulnar artery we imaged the forearm vasculature and he had a large and well developed interosseous artery. I hope this helps.
Samir Pancholy:
Sasko has the largest single center experience. Seems very safe in experienced hands. Agostoni has the “Switch” registry with similar data.
Tejan Patel:
I know before I retire we will be using Ulnar arteries routinely in those situations! For now I go to femoral if good pulse and no significant PVD and use closure device. All those studies are good and encouraging but I still live in USA.
Samir Pancholy:
Great point Tejan, agree. Our fellows grade bleeding risk pre Cath so if bleeding risk not high will go femoral (assuming bilateral UE issues). Biologically Sasko has convinced me. But liability risk (frequently completely frivolous) and outcomes are decided by juries and expert witnesses (of course excluding Ian) with herd mentality.
Ian Gilchrist:
Most common law suits related to cardiac Cath are femoral vascular and access site related by far. Always consider the real risk of a law suit from the femoral vs theoretical from radial and take your choice.
Samir Pancholy:
True as well.
Sasko Kedav:
We have experience in using ipsilateral ulnar artery in cases of occluded radial artery in more than 500 cases. So far, there has not been a single case with hand ischemia. Generally, ulnar artery has much stronger pulsation and is easier for access when radial artery is occluded. Smaller caliber introducers (up to 6F) and meticulous post procedural care (patent hemostasis and bleeding prevention) is highly recommended. However, at this stage without multicenter experience, we cannot generalise this approach in all comers, particularly not for low volume centers.
Tak Kwan:
We also have a smaller experience of “Ipsilateral translunar catheterization in patients with radial artery occlusion”, published in CCI 2012, same conclusion as Sasko.
Ronald Caputo:
I get asked this question very often. It is good to see that there is significant experience with ipsilateral ulnar to justify a decision to use that approach. In the US we are very risk averse and any hand injury is a high damage award situation. Consequently I would be more apt to use the femoral.
Kimberly Skelding:
I concur. This sends me to the femoral.
David Hildick-Smith:
Using the ulnar when the radial is known to be occluded invites disaster. If both the radial and the ulnar become occluded, the patient will be lucky to keep their hand, and if they dont, you will be in deep, deep, do-dos.
Olivier Bertrand:
I beg to disagree David. Experience has shown extensive collaterals (including interosseous) which will protect even if both radial and ulnar get occluded Indeed I am not pretending that there is 0% risk but I do not agree for a significant risk Interesting topic for debate at AIM-RADIAL 2015!!!!
Sunil Rao:
This will be a great debate!! Olivier – make popcorn!
David Hildick-Smith:
What constitutes significant risk when the outcome in question is losing a hand? 1%? 0.5%? 0.25%? 0.125%? None of these is acceptable for me. There are perfectly good arteries in the leg that lead to the heart too, if the radial approach is not possible.
Olivier Bertrand:
David, I simply said significant because, in contrast to your assertion, there is not a single described/known so far of what you present as an evidence. So far as I am concerned we should get close to 0% 🙂 As we are in an era of evidence-based medicine, that means that there is o chance that a trial be ever performed to evaluate whethere there is a risk or not. Yet some series have already been published showing the safety of it as we are left with a opinion-based medicine we could argue for a long time just my 2 cents.
Kintur Sanghvi:
I agree with Dave to make a stronger case for debate at AIM-R. The patient who suffers hand ischemia despite 0.01% odds will have 100% effect of hand ischemia on his life. Its true in America common reason for getting sued until now was femoral access related complications. But it may be just because not enough radials were (are) done in U.S.
Mitchell Krucoff:
Interesting e-dialogue! If both right and left radials are occluded, I would have a long talk with the patient. Odds are if both are occluded they have been through cath many times, and are likely to have a reasonable perspective to discuss femoral risk vs. hand risk. Data from Sasko and others are reassuring but not definitive. have to keep in mind that both RIVAL and SAFE data both support that experienced radialists have very low femoral complication rates. An ironic fact that should factor in. My 2 cents.
Kintur Sanghvi:
Evidence based medicine should be used on a very strong fundamental of the basic science. We are contradicting ourselves. We were arguing RA is a good choice as it is not an end-artery. The level of evidences (single center cross-sectional) to support using UA when ipsilateral RA is occluded is very weak to risk a patients hand. And make sure no one calls me, Tejan or Dave as an expert witness.
James Nolan:
In the circumstances described I would use femoral.
Sasko Kedav:
Ulnar artery is NOT an end-artery in case with occluded radial artery. In all cases with occluded radial artery there are documented collaterals, predominantly from anterior interosseous artery. Brachial and femoral arteries are always end arteries. What constitutes acceptable risk of major femoral vascular complications (including retroperitoneal bleeding in perfectly done femoral puncture) in 85 year old lady with ACS and occluded radial arteries: 0.25% 0.125%?
Sunil Rao:
This will be a great debate!! Olivier – make popcorn!
Tak Kwan:
So far Sasko has shown 500 cases without complications. Is anyone know a case of complication of occluded radial and occluded ulnar yet?
Kintur Sanghvi:
I hope I am not annoying any of my radialists friends. Keeping this important interesting debate on……to me the UA is an end-artery if the RA is occluded. Because if UA is not an end artery because of the interosseous branches than brachial is also not an “end artery” as seen in this case from today. Angio and PCI of RCA was performed through the radial recurrent branch in to axillary artery while the brachial is occluded for years from the previous brachial cut down procedure.
Olivier Bertrand:
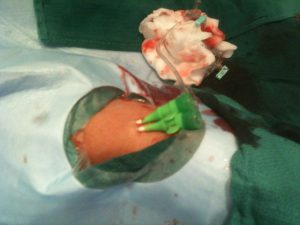
Here is a video and a photo of a patient with occluded right radial and ulnar arteries, we did with Sasko when he taught me transradial carotid stenting. We had to use the left radial after failing to get the radial and we did an injection from the left to prove both occlusions!! (interosseous had grown!) I told Sasko we should publish it as the issue/question would emerge I guess image/video is still worth 1,000 words. Cheers.
James Nolan:
The exact state of the collateral circulation is uncertain in the circumstances of an occlusion of an arm artery. If the femoral is available I can not see why you would not use it, if there is no femoral access the patient is in deep trouble and using an arm artery could be justified. Because Sasko can evaluate these patients and do the procedure safely does not mean the average interventionist can. We should be careful here.
Alejandro Goldsmit:
I vote to make The Debate” in AIM, in one corner…expert favor to use radial and in the other expert corner not agree to use it!!!!
Yves Louvard:
Dear friend, Radial versus femoral in a radial meeting? Are you candidate to support femoral? In UK? (I recommend you not against David Hildick Smith) Ulnar or not when radial is occluded is much better for me.
Josef Ludwig:
Valentine RJ, Modrall JG, Clagett GP. Hand ischemia after radial artery cannulation. J Am Coll Surg. 2005 Jul;201(1):18-22. BACKGROUND: Hand ischemia is a rare but potentially devastating complication of radial artery cannulation for arterial monitoring. The causes and ultimate outcomes of hand ischemia after radial artery cannulation are unclear. STUDY DESIGN: My colleagues and I reviewed the clinical course of radial cannula-induced arterial thrombosis in eight patients during a recent 5-year period. RESULTS: Mean (+/- SD) duration of radial artery ischemia was 3 +/- 2 days. Injuries were associated with advanced (grade IIb) ischemia that affected the entire hand in four patients and first three digits in the other four patients. Radial artery thrombosis was documented using noninvasive tests or arteriography in all patients. Five injuries were initially treated with thrombectomy and patch angioplasty (n = 4) or vein graft interposition (n = 1); two others were treated nonoperatively with vasodilators, and one was observed without treatment. Three of the four patch angioplasty repairs occluded within 24 hours. Regardless of patency, all patients who survived arterial repairs had continuing ischemia that resulted in digital gangrene or amputation. In contrast, gangrene developed in only one patient treated nonoperatively. CONCLUSIONS: These data show that hand ischemia after radial artery cannulation is associated with high risk of tissue loss or amputation. Operative repair offered no advantage over nonoperative therapy in prevention of digital gangrene in this series. We hypothesize that digital gangrene results from distal embolization from the site of the initial arterial thrombosis, producing ischemia that is not remediated by radial artery revascularization. Nonoperative therapy with vasodilators can be equally effective in treating cannula-induced radial artery injuries in some patients.
And let us be fair. Radial is not a religion; Transcath aortic valve replacement t5he pts are > 85yrs. Last week 105. They all have fem. Access and sheath size far above PCI.
Yves Louvard:
Dear all, I am very sorry not to be at AIM radial this year! (Olivier dont forget me next year) Even if I dont need frequently crossover from radial to another approach, I like the Sasko attitude, I am following it since a long time. Of course radial is not a religion but this is the best way to reduce vascular complications, and the best way to make it true for patients is to adapt the technique to perform 100% radial (+ ulnar ?) I will fail, only 97% today. The method is to collect the datas from the cases without any other solutions, analyse them, present or publish, have a controversy in AIM, find the scientific reasons why it is working or not, select, do more. Sasko, I heard many comments from the beginning of the radial ! I remember radial is an elegant approach for treatment of restenosis (1997) probably around 2000 I lost a radial vs femoral controversy in Last Frontier in Interventional Cardiology in Texas 90%/10% recommandations are for people who cannot do this (equipment, volume). We have to go further. It was demonstated for many other topics like LM, multivessel, CTO, bifurcations this is one of the responsibility of our community and AIM Radial ! If we follow recommendations (specially recommendations before official ones), we will not move at all ! Fighting with difficulties make us better even with femoral approach (look also at the BCIS database). Skillness and experience are very important as shown in RIVAL SYNTAX so even if I dont perform Ulnar approach Sasko and others are pioneering, let us look at the datas, let us learn with Sasko dont say this is unethical (remember Josef the 2001 paper refused in JACC without review about 1000 transradial primary PCI). I hope to see the 2 presentations and rebuttal. Please Olivier keep them for me!
Josef Ludwig:
Dear all, I disagree with Yves so far the he and me have learned femoral first. Young operators today start with radial. If radial is 100% how to do interventions requiring femoral. Last week we treated a 105 old pt by TAVI. Not good if they start learnin femoral in this severe ill pts.
Yves Louvard:
But Josef, fellows in our group are begining structural after 2 years! of course not from nothing as we did for radial, with a proctor. And as experienced radialists they will become quickly better than pure femoralists even in femoral approach. The futur interventional cardiologists will learn femoral for TAVI like transseptal for atrial appendage closure (are you still doing transseptal Josef ?)! Nice to discuss again Josef.
Josef Ludwig:
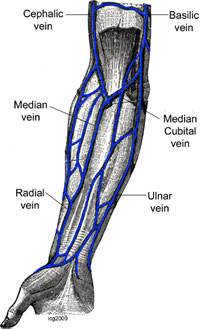
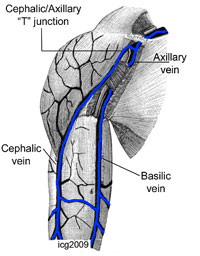
No, because of my neck I am more writing than doing cath work. Dear friends, I remember that I was reading some yrs. ago that anatomic variation of the radial are unilateral in the majority if pts. However, I cannot find the Ref. Can anyone help?
James Nolan:
Josef, I dont think it is well documented, but some variation, such as subclavian tortuosity, is definitely more common on the right than the left and the data for this is in the Italian studies of left v right radial I have also seen something suggesting loops etc. are often unilateral but I dont have the reference to hand
Yves Louvard:
Dear Josef, You heard that in Massy radial meeting at the end of the 90s Congratulations Presentations were done by Dr Rodriguez Niedenfuhr (anatomist). Here are my 2 (one & two) reference papers by him. You will find that there are no rule for bilaterality of vascular anatomical variations in arm/forearm (thousands of autopsy) Best regards.
Ian Gilchrist:
Here are 3 (one, two & three) anatomic articles that discuss asymmetric arterial patterns.
James Nolan:
Heavy going those articles. So, it looks like about 50% are bilateral, 50% unilateral?
Josef Ludwig:
1 study 38%, <50%. Asians may differ.
Tejas & Sanjay:
One should avoid to puncture ulnar.